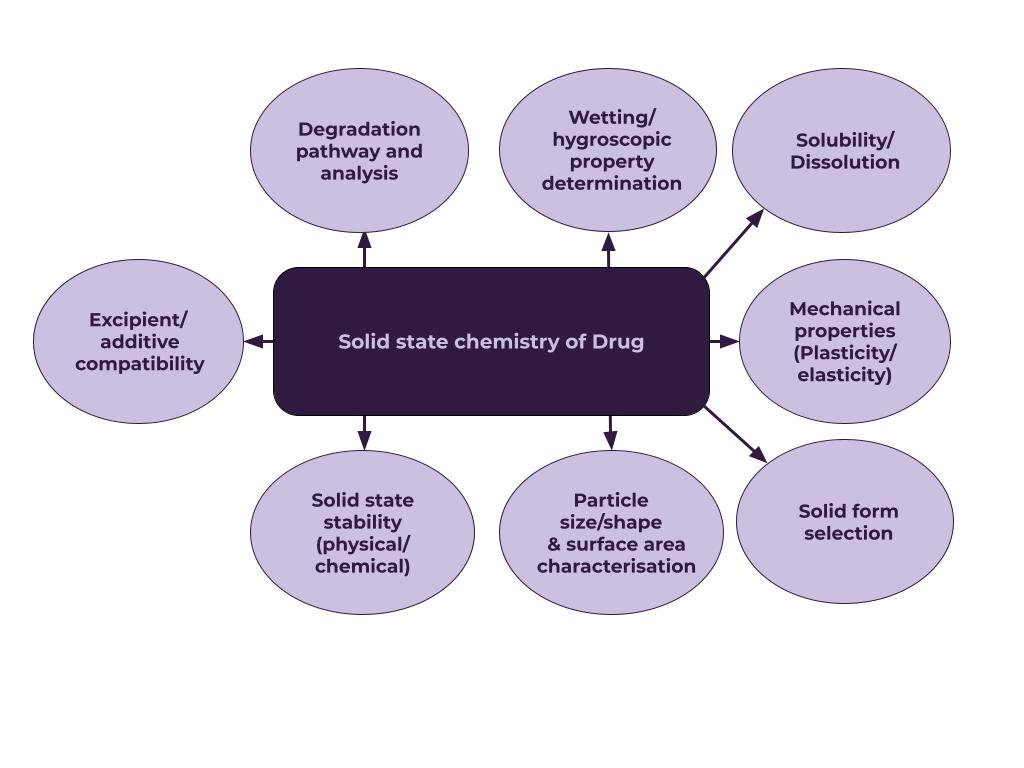
Solid-state Analysis
Are you looking to
- Select a stable, robust, and reproducible drug product?
- Mitigate development risks?
- Set adequate acceptance limits for quality specifications?
Select the stable form as soon as possible and study all-solid-state properties.
Monitor properties of each batch.

For particle/powder analysis, we can offer:
- Examination of particles sizes
- Particle morphology by optical and scanning electron microscopy
- Specific and outer surface area
- Porosity
- Hygroscopicity
- Crystallinity, polymorphic purity
- Thermal properties: melting point, glass transition, heat capacity, sublimation etc.
- Dissolution rate
- Stability
- Density, including bulk and tapped density
- Single-crystal structure determination (non-GMP)
- IR spectra for identification and imaging
- Raman spectroscopy
- Other – please ask