Do you want to convince the authorities and auditors that your method is valid?
We apply the ICH guidelines for method validation.
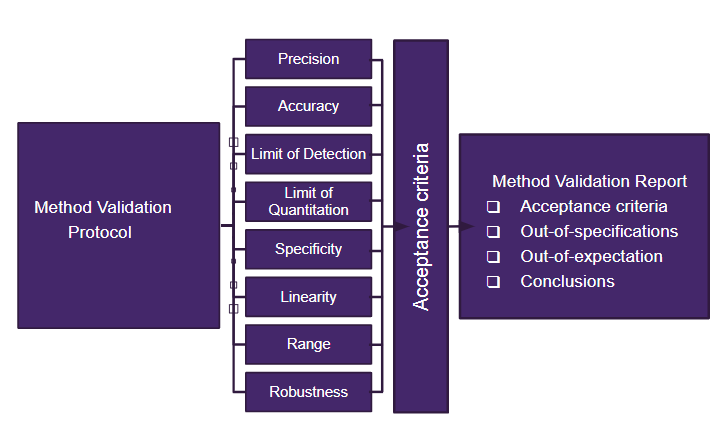
Our validations are customised services. Mix and match between:
- Validation protocol including setting acceptance criteria
- Verification of method suitability
- Verification of the method accuracy and precision, and repeatability
- Robustness (including intermediate precision)
- Conclusions about compliance with acceptance criteria
- The Validation report includes all documentation
All our procedures are compliant with the ICH guideline: Q 2 (R1) Validation of Analytical Procedures: Text and Methodology (europa.eu) and will be customised for each specific technique. We define robustness, repeatability, accuracy, suitability, linearity, LoD and LoQ.

