Method Development
Are you always sure that the analytical (standard) method can give you adequate results when ordering individual analysis?
The optimisation of a method during the manufacturing process development is frequently needed to fit the systematically chosen Quality-By-Design (QbD).
Did you know that
- Most standard methods have exceptions
- Frequently material properties impact the results or even make a method irrelevant
- It saves time and money to adopt standard methods to fit each case
Particle analytical has +20 years of expertise in method adoption and development!
More Information
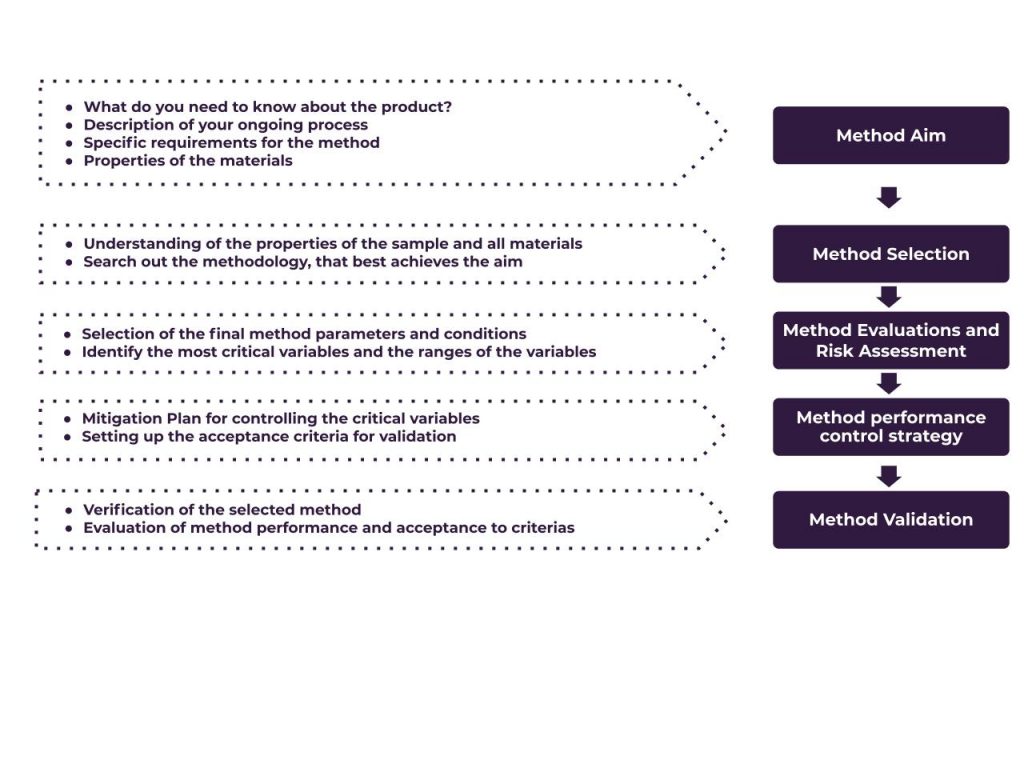
This is an example of a method development process:
- Setting the aim of the method
- Searching for the optimal analytical parameters to achieve the goal
- Study the properties of the material – we define the package in collaboration with you
- Finding the optimal measurement parameters, i.e. measurement time per step, equilibration conditions, pre-drying time, dispersion media and time etc.
- Comparing the method under development with the existing methods
- Evaluating the critical method variables
- Evaluation of method robustness
- Building strategy of risk mitigation
Example of possible Method Development Strategy. The Scheme adopted from Vogt FG, Kord AS (2011).



