Home / Knowledge Hub / Regulatory requirements / ICH requirements
ICH Guidelines
Ensuring compliance with Particle Analytical as your partner
ICH Guidelines - references for the determination of particle sizes
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has defined 14 quality guidelines (Q1 to Q 14), also called ICH Guidelines for the pharmaceutical industry.
The purpose is to define relevant thresholds for impurity testing, and the guidelines define the approach to pharmaceutical quality in the pharmaceutical industry on Good Manufacturing Practice (GMP).
The ICH guidelines are critical in maintaining the highest standards in pharmaceutical development and production. They encompass various aspects, including the quality, safety, and efficacy of medicinal products. For particle analysis, the Q6 A, Q8 and Q9 guidelines are particularly relevant, and is further described below.
Understanding the Q6 A Guideline
ICH Q6A: Test procedures and acceptance criteria for new drug substances and new drug products
ICH Q6A provides a framework for setting specifications and acceptance criteria for new drug substances and drug products.
It emphasizes the importance of controlling particulate matter to ensure the purity and quality of pharmaceuticals.
According to ICH Q6A, particle size distribution and contamination must be rigorously monitored and controlled to meet the required specifications.
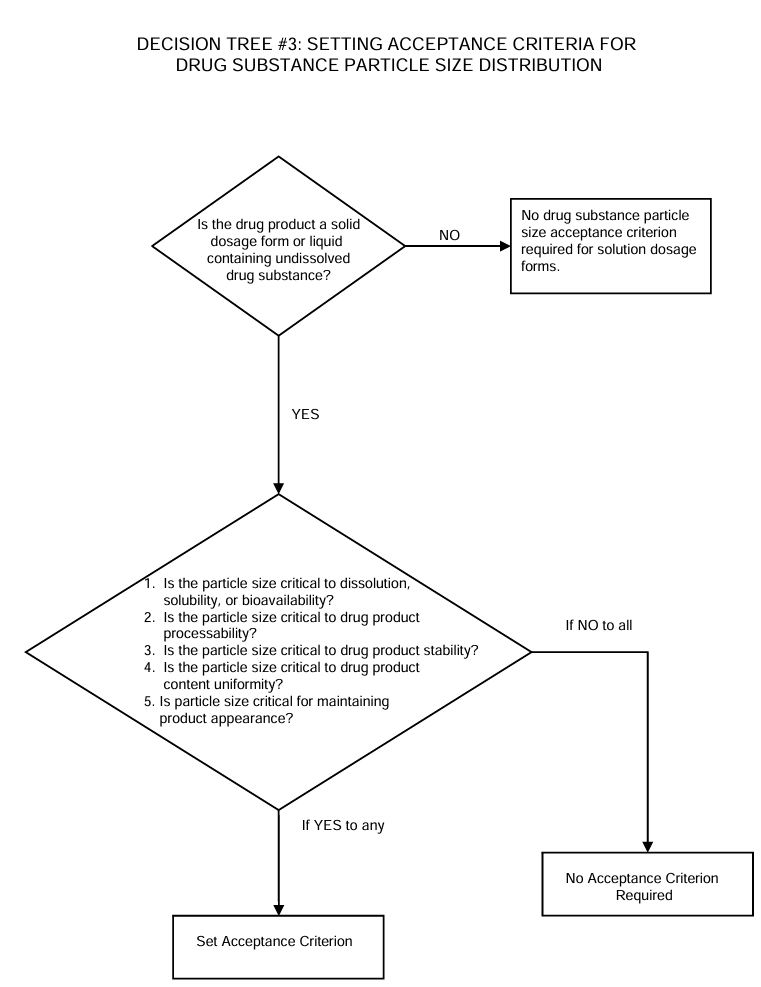
The decision tree #3 describes the potential need for setting particle specifications. It is a helping tool to determine the setting acceptance criteria for drug substance particle size distribution.
Only in theory it is possible to answer “no” to all, thus in practice you need to set acceptance criteria for particle size.
Understanding the Q8 Guideline
ICH Q8: Pharmaceutical Development
ICH Q8 focuses on the development of pharmaceuticals, highlighting the importance of understanding and controlling the manufacturing process to ensure product quality.
This guideline encourages the implementation of Quality by Design (QbD) principles, which involve thorough particle characterization and control as part of the design and development process.
Effective particle analysis is crucial in identifying critical quality attributes (CQAs) and ensuring they are maintained throughout the product lifecycle.
Understanding the Q9 Guideline
ICH Q9: Quality Risk Management
ICH Q9 provides a systematic approach to quality risk management, essential for assessing and controlling risks related to pharmaceutical production.
Particle analysis plays a vital role in this process by identifying potential risks associated with particulate matter.
The level of risk depends on the compound in question. For a highly soluble compound the effect of a slight change in particle size might be very limited, whereas it might have large impact for a poorly soluble drug.
In the guideline you find two primary principles of quality risk management:
- The evaluation of the risk to quality should be based on scientific knowledge, and link to the protection of the patient
- The level of risk should commensurate the level of effort, formality and documentation of the quality risk management process
Please pick a subject
Knowledge Hub
Explore our Knowledge Hub for regulatory guidelines, publications, events and FAQ, that bring you insights into particle analysis
How we can help
Learn more about How we can help you addressing problems in your development or manufacturing process, by applying our strong technical and analytical skills.
Why choose us
By choosing Particle Analytical, you get a strong and specialized partner, with a wide range of analysis, scalable and flexible services, and strong accreditations.